Energy & Work
"Energy" is a word you've heard before. Countries worry about getting enough energy (mostly in the form of coal, oil, and natural gas) for their citizens, and access to energy sources leads to geopolitical drama and even wars. We dig holes in the ground (mines) to get coal, and drill for oil on land and on the sea floor. Our use of most energy sources causes pollution and global climate change. Oil tanker accidents, pipeline breaks and oil rig disasters kill animals and ruin ecosystems. The cost of energy can sometimes rise and fall dramatically, causing chaos in the economy and hardship to real people who, when prices spike, might not be able to afford gasoline for their cars or fuel to heat their homes in winter. Even if you are lucky enough to be able to afford the energy your family needs, it is still a significant part of the family budget. Nuclear energy is controversial, especially after the (relatively minor) accident at the Three-Mile Island nuclear power plant in Pennsylvania (in 1979), and the disasters at Chernobyl, Ukraine (1986) and Fukushima, Japan (2011).
We go through all this to get this mysterious thing we call energy. So, what the heck is energy? Energy is one of the most important concepts in physics, all of science really, but is surprisingly difficult to define.
Energy is a numerical quantity associated with a system, and is conserved. (That means that energy is never created not destroyed, but merely changes from one form to another.) This property of conservation serves, essentially, as a definition of energy. The fact that energy is conserved allows us to calculate the behavior of a system while ignoring many details.
Why is the concept of energy so useful? Because energy is a scalar (unlike force and momentum, which are vectors) so energy is mathematically simpler to deal with.
Kinds of energy: Here are brief descriptions of the kinds of energy we’ll be discussing most often.
- kinetic energy = KE = the energy an object has because of its speed
- potential energy = PE = the energy an object has because of its position relative to external forces. There are different kinds of potential energy, the most important of which are gravitational potential energy, elastic potential energy, and electric potential energy.
- chemical energy = energy contained in the chemical bonds of molecules. The gasoline that powers cars and the food that powers us are examples.
- electromagnetic energy = energy contained in electric and magnetic fields and electromagnetic waves such as visible light, x-rays and radio waves
- thermal energy = Q = the sum of kinetic energy of an object's atoms and molecules, which are always moving or jiggling. Faster = hotter. Sometimes thermal energy is called internal energy or heat.
- mass-energy = energy matter has because it has mass. This is what the famous equation
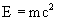 is about.
is about.
We’ll talk about each of these forms of energy in more detail later. In the meanwhile, here's a song about energy.
You can think of energy like coffee; it can be poured from one mug into another, but the total amount isn't changed by pouring it. (In this analogy, no one drinks the coffee.) If some is spilled, the spillage represents energy that becomes the part of the random thermal vibrations of the atoms and molecules of the object and environment. That energy is "lost" in the sense that it is no longer easily available to do useful work.

LAW OF CONSERVATION OF ENERGY: Energy is never created
nor destroyed — it only changes forms.
How do these energy transformations take place? One way is through work.
Work is defined as the component of force in the direction of motion, multiplied by the distance the object moves. In equation form,
![]()
This is one of those situations where a word in common, everyday usage has a meaning that differs from the technical definition intended when physicists use the word. For example, if someone stands while holding a very heavy rock, no work is being done because there is no motion. It doesn't matter how heavy the rock is or how much effort it takes to hold up the rock. Picking up the rock, lifting it from the ground to a higher location, that is work, but just holding it in place is not.
The work is defined as a force times a distance, the standard SI unit of work is the unit of force (Newtons) times the unit of distance (meters).
![]()
Because this is the unit of work and also all forms of energy, we will need to use it a lot. The scientific community has therefore given it a shorter, easier name: the Joule (symbol: J). The name is in honor of James Joule, a 19th-century English scientist and brewer who, in the mid- and late-1800s, studied the relationship between heat and work. His experiments led to the law of energy conservation.
Other energy units
The units of energy and work are any unit of force times any unit of distance. In English units, the usual unit used is the foot·pound. One ft·lb equals 1.36 J
Some other energy units are defined based on how much energy is needed to raise the temperature of some substance, usually water. A calorie is the energy needed to raise one gram of water 1 Celsius degree. One gram of water is about one cubic centimeter. It turns out that it takes about 4.186 Joules to heat 1 gram of water one degree, so
1 calorie = 4.186 J
In the context of food and the labels used for consumer information, the kilocalorie is used. Confusingly, in the U.S. this is usually just called a calorie. Some people will capitalize the word Calorie to mean 4186 J instead of 4.186 J, but that is not universally followed. To keep these straight, I will call them the "calorie" and the "food calorie", respectively.
1 food calorie = 4186 J
Another unit of energy you might hear mentioned is the British Thermal Unit, more commonly known as a BTU. It is the amount of energy needed to heat one pound of water by one Fahrenheit degree. One BTU is about 1055 Joules.
Lastly, there's another unit of energy called the kilowatt·hour, usually abbreviated as kW·hr or KWH. This is quite likely the unit used on the energy bill for your home. We'll talk about how the kW·hr is defined after learning what a watt is, but in the meantime, for the sake of completeness here, know that
1 kW·hr = 3.6×106 J = 3.6 MJ
1. Here is an online ad for a gas-powered grill. With the burners on, at what rate does it generate heat in Joules per hour? In Joules per second?
Food Labels Around the World
In most countries other than the U.S., the energy content of food is actually labeled as such: notice the "contenido energetico" label on this box of breakfast cereal from South America. The energy content is given in both kilocalories ("food calories") and kilojoules.
Here's a beverage bottle from Australia. Energy is given in standard SI units.