Atoms & States of Matter
Atoms Imagine you have a piece of copper. If you cut it in half, what do you get? Well, you get two (smaller) pieces of copper, of course! But suppose you took one of those small pieces and chopped it in half. You'd have even smaller pieces of copper. But could you keep doing that forever, cutting the smaller and smaller pieces in half and always have copper? This was the question asked by Leucippus and Democritus, Greek philosophers of the 5th century BC. Although they had no evidence that we would today call scientific, they felt that the answer must be no. There had to be a point at which you can't cut the copper in half anymore. They called the smallest-possible piece of anything an atom, from the Greek word-roots a-, meaning 'no, not, without', and tomos, 'cutting, split'. They felt that the atom could not be cut because there would be no space inside it. They knew that atoms, if they existed, must be smaller than can be seen with the eye. This made it difficult to actually prove they existed.
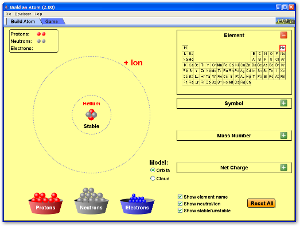
Atoms are really small. For example, a single drop of sea water contains 50 billion atoms of gold. You would need to extract all the gold atoms out of 200 tons of water to get enough gold to make a tiny speck large enough to barely see with your naked eye. ETYMOLOGY: Other words that use the a- root include amoral (without morals) and asymmetrical (not symmetrical). Some words incorporating the root -tom include tonsilectomy and appendectomy, operations in which the tonsils or appendix are cut out of the body. The existence of atoms wasn't definitively shown until the 1800s, and there were no direct pictures until the 1980s. Here (to the right) is a picture of silicon atoms in a solid crystal. In the 1800s it was known that the atom had to be made of positive parts and negative parts, but the electron was not discovered (by J.J.Thompson) until 1899, the nucleus (by Ernest Rutherford, using the gold-foil experiment) in 1911. Despite what Democritus thought, atoms are 99.999,999,999,999% empty space. The nucleus contains protons (positive charge) and neutrons (no charge). Protons and neutrons have almost identical masses, and each has over 1800 times the mass of an electron. Because they reside in the nucleus, protons and neutrons are together called nucleons.
|
 |
Surrounding the nucleus is the electron cloud (negative charge). The radius of a proton or neutron is about 1x10-15m. The radius of the H electron cloud is 0.5x10-10m; for the largest atoms the radius is about three times as much. For Hydrogen, ratio is 50,000 of nuclear and electron cloud radii. For a tennis ball proton model (radius 3 cm), the electron of the Hydrogen atom is 50,000 times 3 cm away, or 1.5 km (roughly a mile). Size of the electron itself is very small, so small that its size has never been measured.
There are 92 elements that occur naturally. They differ in how many protons they have in their nucleus (and to neutralize that positive charge, they have the same number of electrons in orbit around the nucleus.) Hydrogen is the simplest, with only one proton; Uranium has 92 protons. Elements with > 92 protons are artificial, created in nuclear reactors or particle accelerators. The largest artificial atoms have about 112 protons in the nucleus.
atomic number = # of protons
mass number = # of protons and neutron = # of nucleons
Atoms of the same element can have differing numbers of neutrons. These differing versions of a given element are called isotopes. For example, carbon atoms have 6 protons in the nucleus, and therefore 6 electrons orbiting the nucleus, making the entire atom neutral. The number and energies of the electrons determine the chemical properties of carbon: its melting and boiling points, what other atoms it bonds with (or doesn't), etc. Most carbon atoms also have 6 neutrons in the nucleus, keeping the protons company. About 1% of carbon atoms, however, have 7 neutrons, and an even smaller percentage have 8 neutrons. The extra neutrons have virtually no effect on the chemical behavior of the carbon. To distinguish between the different isotopes of an element, the mass number is often said after the element name. For example, most carbon atoms are Carbon-12 (6 protons, 6 neutrons), but the carbon atoms with one extra neutron are called Carbon-13, and those with 2 extra neutrons are Carbon-14.
The periodic table lists all the elements in order of atomic number, the number of protons in the nucleus, which equals the number of electrons in the electron cloud. The electron cloud has differing energy levels or shells, but we aren't going to go into detail about those here. The elements grouped in vertical columns of the periodic table have the same number of electrons in the outermost shell, and so those elements have very similar chemical properties.
Atoms can bond (stick) to each other, forming molecules. Atoms and molecules are both called particles.
Activities & Practice
to do as you read
1. Listen to the Elements Song by Tom Lehrer. Here's another version.
2. The radioactive isotope Polonium-210 was used to murder former KGB agent Alexander Litvinyenko in 2006. Find Polonium on the periodic table. How many protons does it have? How many neutrons does Polonium-210 have?
3. Name some elements that have similar chemical properties to Carbon.
4. Check out this website that graphically illustrates the different isotopes of the first ten elements. Many isotopes are radioactive (unstable), meaning that protons can spontaneously turn into neutrons (or vice versa), changing the identity of the atom.
http://www.colorado.edu/physics/2000/applets/iso.html
The arrangement of nuclei as in the above applet is call a nuclide chart. The one above only goes up to atomic number 10 (Neon). A complete version is much more impressive, such as the National Nuclear Data Center's Interactive Chart of the Nuclides.
STATES OF MATTER: There are five so-called states of matter. The three you are most familiar with are solids, liquids, and gases.
- Solids are made of particles (atoms and molecules) that are attached to each other, usually in a regular arrangement called a crystal. Solids maintain their volume and shape because the particles are locked to each other and can't move much or switch places.
- Liquids maintain their volume but not their shape. The particles are still attached to each other, but more loosely so they can flow and switch places.
- Gases are made of particles that are not attached to each other at all. Instead, they move freely and bounce off each other when they collide. Gases maintain neither their shape nor volume: a gas can be squeezed under pressure and change its volume.
|
 |
Two states of matter that are less familiar are
- Plasma. A plasma is a gas that is hot enough that
the atoms collide with each other so violently that electrons are knocked
free from the
atoms. In other words, the gas is ionized. The plasma
is a mixture of positively-charged ions and loose electrons. Plasmas
with which you are familiar include:
- The large electrical discharges (sparks) in the atmosphere that we call lightning raise the temperature of the air through which it passes to over 30,000°C, stripping electrons from the atoms in the air and creating (for a brief time) a plasma.
- The gases inside a fluorescent light
- Arc welding
- Plasma TVs
Although plasmas are relatively rare in your everyday experience, most of the matter in the Universe is in the form of plasma. Stars are almost entirely ionized gas (albeit very, very dense in the center due to the crushing force of gravity), and most of the (very, very thin) gas that permeates interplanetary and interstellar space is also ionized.
- Bose-Einstein Condensate. A Bose-Einstein condenstate (BEC) is a state of matter that occurs when some atoms are cooled to a couple billionths of a degree above absolute zero. Essentially, the atoms lose their individual identities, forming a single blob. We'll discuss this more when we talk about Quantum Mechanics. BECs are named after Satyendra Nath Bose, an Indian physicist, and Albert Einstein, the famous German physicist. In the 1920s they were collaborating in the new field of quantum mechanics when they predicted the existence of this state of matter, but at the time there wasn't the technology to cool a sample of atoms so close to absolute zero. The first BEC was created in a lab in 1995 by Eric Cornell and Carl Wieman at NIST and the University of Colorado.
The processes of solidification, deposition or condensation all require the release or extraction of thermal energy (heat) from the material. The addition of heat to a substance can cause the opposite changes, namely melting, sublimation and evaporation, respectively. Here's a video showing heat released when a liquid solidifies.
5. Read more about BECs at http://www.colorado.edu/physics/2000/bec/what_is_it.html
Additional Activities & Practice
6. The symbol used for the metal tungsten is W.
(a) Find tungsten on the periodic table. What is its atomic number?
(b) Two isotopes of tungsten are W-182 and W-184. By studying the ratio of W-182/W-184 abundances in the mantles of the Earth and Moon, astronomers believe they have determined that the Moon formed between 60 and 100 million years after the Earth formed. (It's a long chain of reasoning that we won't go into now. See Physics Today, Feb 2008 for details.) How many protons and neutrons do W-182 and W-184 each have?