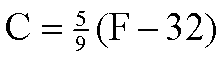Temperature
The atoms and molecules that make up matter are always moving. If the matter is a plasma or gas, the particles move around more or less independently of each other, except of course when they collide. In a liquid, the particles are strongly attracted to each other, but are still free enough to move around and flow around each other. In a solid, the particles are attracted so strongly to each other that they are locked together, often in a repeating pattern called a crystal. Even when in a crystal, the particles can jiggle about, like a rabid dog tied to a tree.
All the particles in a certain piece of matter are not moving with the same speed. In general, the lighter particles move faster than the heavier ones, but on average the kinetic energy of the particles is the same regardless of their mass. The more thermal energy the material has, the more KE the particles have. Temperature is an indication of this average kinetic energy. For air at room temperature, the average KE of the moving air molecules is about 6x10-21Joules. In fact, we could use joules as our unit of temperature if we wanted to, but as you can see, the numbers involved would usually be very small. We choose to use temperature scales instead, the most common of which are known by their inventors' names: Fahrenheit, Celsius and Kelvin. Every such scale must have at least two reference temperatures which are used to define it.
Activities & Practice
to do as you read
That temperature is an indication of how energetically atoms and molecules are moving was first proposed by James Joule in 1843, but it was not accepted initially. The prevailing view at the time was that heat was a fluid substance (called "caloric") which could flow from one object to another.
- The Fahrenheit scale was invented by one Gabriel Fahrenheit, in 1724. The reference temperatures used were a water/ice/salt mixture, and human body temperature. He chose to make human body temperature equal to 96 degrees, but later adjustments ended up making body temperature 99 degrees instead.
- Anders Celsius chose instead to use the freezing and boiling points of water (at average sea-level atmospheric pressure) as the reference points for his scale, and set those point equal to 0 and 100 degrees, respectively.
- Since temperature is an indication of the kinetic energy of atoms and molecules, logically there must be a coldest temperature, where the KE is zero and the particles are not moving. It seemed like a good idea to William Thomson, in 1848, to design a absolute temperature scale that started with 0 at this coldest possible temperature, the so-called absolute zero. His proposed system used degrees of the same size as in the Celsius scale (i.e. there are 100 degrees between the freezing and boiling points of water), but the whole number line is shifted, so it counts up from absolute zero. There is no such thing as a negative temperature on Thomson's scale. After Thomson was elevated to the English peerage in 1892 in tribute to his many scientific achievements, becoming the 1st Baron (Lord) Kelvin, his temperature system came to be known as the Kelvin temperature scale.
Here are some key temperatures on the three common temperature scales.
| absolute zero | liquid Nitrogen | freezing point of water | "Room temperature" | human body temperature | boiling point of water (at 1 atm) | |
|---|---|---|---|---|---|---|
| Fahrenheit | -460 |
-321 |
32 |
68 |
99 |
212 |
| Celsius | -273 |
-196 |
0 |
20 |
37 |
100 |
| Kelvin | 0 |
77 |
273 |
293 |
310 |
373 |
The equation converting between Celsius and Fahrenheit is...
The (F-32) term is the number of Fahrenheit degrees above the freezing point of water. The fraction 5/9 is the ratio of the size of a Fahrenheit degree to a Celsius degree: there are 100 Celsius degrees between the freezing and boiling points of water, and 180 (i.e. 212-32) Fahrenheit degrees. That's a ratio of 5/9.
Additional Activities & Practice
1. The temperature at the center of the Sun is about 15 million degrees Kelvin. What is this temperature in Celsius and Fahrenheit?
2. The temperature of the cosmic microwave background, a radio glow left over from the Big Bang that pervades the Universe, is 2.7 K. What is this temperature in Celsius and Fahrenheit?
3. The classic Ray Bradbury novel Fahrenheit 451 was about a society where books are banned, and in fact burned. The title is a reference to the temperature at which paper will catch fire. What is 451°F in the Kelvin and Celsius temperature scales?
4. There is one temperature at which the Celsius and Fahrenheit values are equal. What is that temperature?
5. What is the equation for Fahrenheit temperature in terms of the Celsius temperature?
6. If you graph Celsius temperature as a function of Fahrenheit temperature, what shape is the graph?
7. A superconductor is a material that conducts electricity with no resistance at all. Not even a little bit. Superconductors aren't used in common appliances because they require cold temperatures. The highest temperature at which any material superconducts is -138 °C. What is that, in Fahrenheit and Kelvin?
References:
"William Thomson: King of Victorian physics" (http://physicsworld.com/cws/article/print/16484; accessed 10Jan2009)