Degrees of Knowing
How confident are you in the following claims? Mark whether you think each idea is TRUE or FALSE, and also how confident you are in that opinion.
The central tenet of science is that all claims should be possible to test. It doesn't matter what some expert says, or an ancient book. The only thing that matters is what Nature says, and we ask questions of Nature by conducting experiments and observations.
The most stringent test is falsifiability: the notion that any hypothesis you come up with to explain the workings of Nature has to be amenable to being proved false. That is, the hypothesis has to be able to predict the behavior of a real experiment or a result of an actual observation. If that experiment is performed and the prediction doesn’t match what really happens, then there are several possibilities: (1) the experiment was done wrong, (2) the whole hypothesis is hogwash, or (3) the hypothesis is partially correct but can be modified to make it better. We redo the experiment and double-check all our calculations to make sure we haven't made some silly mistake. (Mistakes happen all the time.) Then if things still don’t check out, the hypothesis must be modified or pitched. If the hypothesis correctly predicts and explains the results of the experiment, the hypothesis can be upgraded in stature to theory. (In practice, the word ‘theory’ is often used rather loosely, and is often applied to unsupported hypotheses when it shouldn’t.) The more tests the theory passes, the more and more confident we feel in that theory. Do we ever feel absolutely 100% confident? No. There are "degrees of knowing", varying levels of confidence, but we are never really sure. Only in pure mathematics (like item #1 above), not science, can absolute Truth be established.
An example of a scientific hypothesis is this: “Heavy objects fall to the ground faster than light objects.” This is #3 on the list above. Aristotle made this claim in the fourth century BCE. It is ‘scientific’ because it is possible (and rather easy, in this case) to check this statement to see if it is wrong or not. It is falsifiable. But no one performed a test of the statement until twenty centuries later, when Galileo dropped two balls of differing weights off the Leaning Tower of Pisa. They hit the ground simultaneously, proving the hypothesis wrong. (Actually, it is historically unclear whether Galileo himself actually dropped the balls, or whether a friend or colleague did it on his behalf. Either way, he directed and interpreted the experiment.)
LEFT: Aristotle, and the Leaning Tower of Pisa, with Galileo.
Why did it take so long for someone to check the hypothesis? First, it seems so obviously true. If you simultaneously drop a feather and a rock, the rock hits the ground first. Secondly, Aristotle was a philosopher of such stature that to question him was considered akin to sacrilege. It was Galileo’s disproof of that centuries-old belief that bolstered the new discipline called ‘science’ and established its rules: always perform an experiment to verify your hypothesis, and accept its judgment even if it violates your expectations, your ego, or the statements of some authority. (Feathers fall slower than rocks because of air resistance. If you make the air resistance insignificant, by dropping two heavy but different-weight objects, or by dropping them in a vacuum, you'll see they fall at the same rate.)
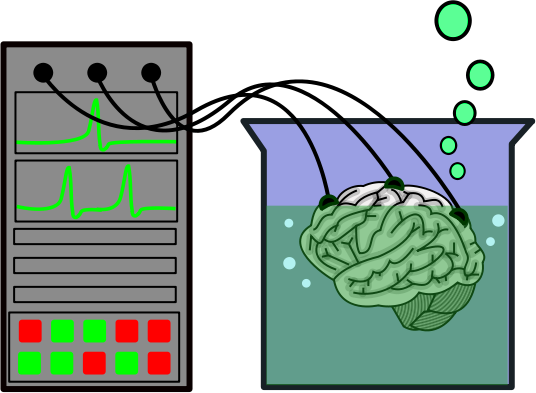
An example of a non-scientific idea is the brain-in-a-vat hypothesis, which is this: The Universe has no physical reality, and neither do we. All people exist only as brains, and all of our experience is merely illusion, piped into our brains as if we had real eyes and ears. This idea was used in the Matrix series of movies. This can be taken one step further, to extreme solipsism: There is only one conscious being in the Universe, namely me. Everything around me, including other people, are only illusion and do not really exist. Neither version of this idea is scientific, because there is no way to disprove it. Say you performed an experiment that you thought disproved the brain-in-a-vat hypothesis. A proponent of the idea would simply say that the experiment itself was an illusion, and thus its results are meaningless. So in fact it is possible that the Universe has no physical existence and is mere illusion, but we’ll never be able to prove it false.
ABOVE LEFT: A brain in a vat, being fed sensory information.
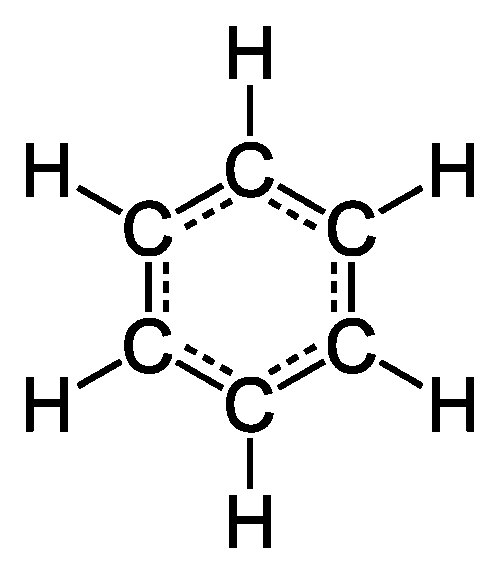
ABOVE RIGHT: A benzene ring, the structure of which came to chemist Kekule in a dream.
Falsifiability is both constraining and liberating. Constraining, because if your theory doesn’t agree with experiment, it has to be changed or abandoned, no matter how cherished that theory. But falsifiability is also liberating, because it doesn’t matter how you come up with your theory — years of mathematical self-flagellation, or a burst of inspiration. The theory either withstands the experimental test or it doesn’t; where the theory came from is irrelevant. You could literally dream up the solution to a mystery. That’s what Friedrich August Kekulé did in 1865. The German chemist was trying to solve a conundrum facing the young field of organic chemistry: benzene (C6H6) was known to have six carbon atoms and six hydrogen atoms. Kekulé himself had shown, in 1858, that carbon was tetravalent, meaning each carbon could form four bonds. Carbon atoms freely bond to each other and form long chains, to which other atoms may bond. This understanding went a long way to explaining, via structure, the bewildering variety of organic compounds. The equal number of carbon atoms and hydrogen atoms in benzene, though, presented a problem. If benzene were a simple hydrocarbon chain, there would be leftover bonds and benzene would be a very reactive chemical. In actuality, benzene is not so reactive. Kekulé, while dozing in a horse-drawn bus, dreamt of a snake biting its own tail whilst whirling about. The benzene molecule, he realized, is a ring rather than a chain. Those extra bonds are utilized in closing the loop, and the puzzle was solved. “Let us learn to dream,...”, he later wrote, “but let us also beware of publishing our dreams until they have been examined by the wakened mind.” Kekule’s laboratory work largely confirmed his dream, although some subtleties had to await the development of quantum mechanics some sixty years later.1
Notice that falsifiability means that you have to be able to prove a hypothesis is false, using experimental tests. This is not the same as proving the hypothesis is true. In fact, no hypothesis or theory can ever be The Truth. Instead, they are mental and mathematical models that approximate reality (whatever that is), but can never correspond exactly to that reality. A most appropriate example (and one which we’ll delve into in great detail shortly) is Newtonian mechanics. In the mid-seventeenth century Sir Isaac Newton essentially invented mathematical physics. He invented calculus along the way, as a tool for his study of falling and orbiting bodies. The laws he elucidated (such as F=ma) were considered The Truth for two and a half centuries. Why? Because those laws mathematically predicted the paths of orbiting planets, and the motion of earthly objects as well, to the accuracy of the experimental measurements then available. Only in the 1850s did the first serious mystery arise that Newton’s equations could not explain: the odd orbit of Mercury around the Sun2. According to Newton’s laws, a lone planet in orbit around the Sun takes the path of an ellipse, and it should retrace the same ellipse over and over again. But Mercury doesn’t do this — its orbit slowly rotates, or precesses. Actually, because of the gravitational pull of the other planets, one would expect the orbit to precess about 1.542 degrees per century. But Mercury precesses 0.012 degrees/century more than this, and this excess can’t be accounted for with Newtonian gravity. This discrepancy in the precession took a long time to notice, because making that kind of accurate orbit measurement required the advance of technology, and the accumulation of data, for many decades after Newton’s time. Astronomer U.J.Leverrier hypothesized that another planet existed, inside the orbit of Mercury. He even named it: Vulcan, after the Roman god of fire and metallurgy. This unseen planet's gravity must be causing the troublesome 0.012 degree/century precession of Mercury, went the reasoning. This is a very sound idea — Neptune was discovered, by Leverrier in 1846, because he noticed its gravitational effect on the orbit of Uranus. Several astronomers, amateur and professional, believed that they saw Vulcan pass in front of the Sun, but these sporadic sightings were never consistent with each other and the idea of Vulcan eventually died out. Other subtle difficulties with Newton’s Laws arose in the later nineteenth century, but Newton’s physics was so well entrenched that the validity of Newton’s Laws was not questioned. No one thought to.

That is, until Albert Einstein developed the Special and General Theories of Relativity, in the early years of the 20th century. In a sense, Einstein showed that Newton’s theory was ‘wrong.’ Einstein's relativity, for example, fully explains the precession of Mercury's orbit — there is no reason to suspect another planet such as Vulcan as the cause. And yet, Newton’s mechanics is still used for launching rockets into orbit, sending space probes to other planets, designing buildings here on the earth, etc. Newton’s physics only begins to be inaccurate in extreme situations: in the intense gravity near the Sun (as Mercury is), at speeds close to that of light, and within the atom or the nucleus. It is still more than accurate enough for everyday situations, and is still used because it is (mathematically and conceptually) simpler than relativity and quantum mechanics.
Newton’s physics is therefore an approximation to reality. Einstein’s theories of relativity are also approximations to reality — more accurate than Newton’s, but we should not believe that Einstein’s physics are The Truth. It is likely that eventually extreme physical conditions will be found (perhaps inside black holes) where even Einstein’s equations begin to break down. In the course of this book, we will see this happen again and again. Technology is often instrumental in this process. New technology makes different experiments possible that may highlight the shortcomings of current understanding.
So rather than saying Newton discovered his laws, perhaps it is better to say he invented them. Ditto for Einstein and the inventors of Quantum Mechanics.
Another example of an unscientific idea is the existence of God. This is not to say that God does not exist, or that God does exist — merely that the existence of God is not susceptible to experimental test. On one point atheists and believers agree — religion is a matter of faith, not of proof.
There is a place for faith in science. Specifically, it is impossible for every scientist to reproduce every experiment done by others, not even the important ones. Life is too short. But each critical result must be confirmed by someone other than the original discoverers — preferably by several scientists or groups of scientists. It's a community effort, and scientists have faith in that process. When a totally startling discovery is made, the scientific community rightly withholds judgment until confirmation (or rebuttal) is made by others reproducing the experiment. This protects against error and fraud. There have been a number of cases of scientists making up data to support the conclusions they wanted to reach. These cases are rare and doomed to discovery. The whole process doesn't run smoothly, but it is self-correcting. This is why (I assume) you chose TRUE for items 4 and 5 in the list at the top of this page. You may have seen cells (item #4) through a microscope yourself, and so you might have direct experience with that. But (again, I assume) you have never used any of the multi-million-dollar scanning tunneling microscopes that have, in the last few decades, allowed humanity to "see" individual atoms (item #5). Even before the invention of those microscopes, there are many reasons scientists firmly believed in atoms, and you'll learn about those reasons in a future chemistry class. But, if someone somehow came up with a reason for not believing in atoms, you can bet you'd hear about it.
The self-correcting process of science is illustrated by cold fusion, an affair in 1988 widely perceived by the public as a fiasco, but really it's exactly how science should work. Two well-respected chemists (Pons and Fleischman) of the University of Utah claimed to have created nuclear fusion reactions of deuterium in a crude laboratory setup, involving a palladium metal catalyst and a plastic Rubbermaid™ tub. We'll discuss nuclear fusion much later, but the jist is this: for two nuclei to fuse together and form a new, heavier nucleus, the nuclei must be brought extremely close together. This is difficult because the electromagnetic repulsion of the nuclei (which have positive charge) tends to force them apart. How can they get close enough? The only way, it was thought, was to have the temperature extremely high — several million degrees. The momentum due to their energetic thermal motion would overcome the repulsion when they collide and allow the nuclei to fuse together. Why is anyone interested in this? Because the fusion reaction releases large quantities of energy: this is how stars (including the Sun) and thermonuclear weapons work. Thermonuclear bombs create the high temperatures necessary for fusion by detonating a fission bomb next to the fusion fuel. Present-day nuclear power plants work by fission, splitting uranium or plutonium. Various groups of scientists and engineers have been working for decades to develop a fusion power plant. The trick is not so much creating the requisite temperatures (lasers can do the job nicely) but containing the fusion fuel. Any material that touches the multi-million-degree plasma is vaporized, so the 'walls' of the reaction chamber must be non-material, like electric and magnetic fields. The technology is, to say the least, horrendously complicated. So far, only one research reactor has produced more energy than went into the heating and confinement, and that only for a fraction of a second. If eventually perfected, fusion nuclear power plants will be extremely expensive to build but the payoff will be big: no pollution and the fuel will be seawater. Humanity's energy needs would be met, permanently.
So now you can understand the shock and excitement at Pons and Fleischman's announcement that they had created fusion reactions in a tabletop experiment: a block of palladium metal in a heavy-water bath! They believed that deuterium atoms trapped between the palladium atoms were forced together by the crystal structure of the catalyst — close enough to fuse. At room temperature the atoms are of course not ionized: in order to fuse, two deuterium atoms must be forced to overcome the electromagnetic repulsion of their electron clouds as well as of the nuclei themselves. The very idea seems ludicrous to a physicist, but many great discoveries start out as ludicrous ideas.
If there was anything improper in the whole affair, it was how Pons and Fleischman announced their apparent discovery. Instead of issuing a paper to a refereed scientific journal, to be judged by other scientists, they held a press conference. The story made headlines worldwide, as befitted a seeming revolutionary discovery by seasoned scientists. Gleaning the sketchy details from the newpaper accounts, other chemists and physicists around the world tried to recreate the experiment. A few groups saw some energy release, but nothing near what a fusion reaction should produce. There were also no neutrons detected, which would be a byproduct of the fusion reaction. (Pons and Fleischman didn't have any neutron detectors.) The scientific community quickly reached a consensus: although there might be some unusual chemical reaction taking place, no nuclear reactions were happening. Cold fusion was dead. We'll have to wait until the technology of high-temperature fusion is developed enough to be economically viable. In fifty years, maybe.
Activities & Practice
to do as you read
Additional Activities & Practice
1. In a paragraph, describe why your answers were what they were, for items #6 and #7 at the top of the page. What is your "degree of knowing" for those?
2. Describe a test that could be performed to disprove item #8.
3. Describe some idea that you feel confident is true. Is it falsifiable? Describe some experiment that could test the idea.
References
1.William H. Brock, Norton History of Chemistry, 1992 and Isaac Asimov, Chronology of Science and Discovery, 1989
2. U.J.Leverrier, Ann. Obs. Paris, 5 (1859)